- #1
zenterix
- 423
- 60
- Homework Statement
- I'm solving a problem set from MIT OCW's "Principles of Chemical Science". The topic is valence bond theory.
There is a problem that asks us to identify the hybridization of the middle nitrogen atom in the molecule
##CH_3NNN##
- Relevant Equations
- The process I usually go through is to draw a lewis structure, use VSEPR to find the electron arrangement and the molecular shape, and then based on the number of atoms and lone pairs on an atom I choose a hybridization.
The problem is that I am not sure how the ##NNN## portion of the molecule fits together.
After looking on Wikipedia, it seems the structure is
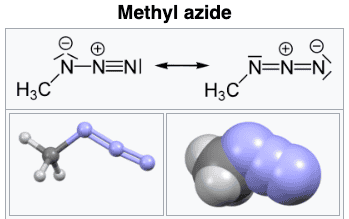
I am not sure about the reasoning that leads one to the above (resonant?) structures. Let's call the leftmost structure A and the rightmost structure B.
My question is what is the hybridization of the middle nitrogen and how do we think about the distribution of electrons among the molecular orbitals.
A nitrogen alone has the valence electron configuration
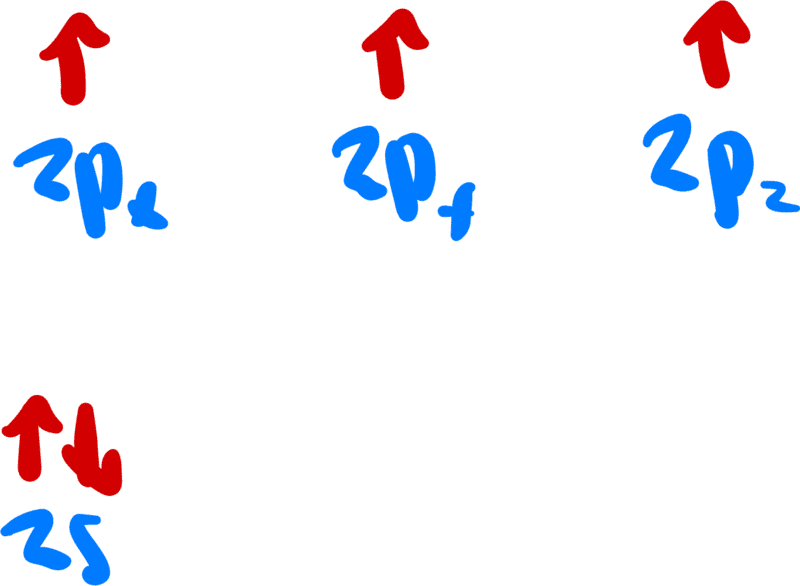
Now, in both the structures, there is are no unpaired electrons or any lone pairs on the middle nitrogen because, for some reason that I am unaware of, one of the electrons on the middle nitrogen goes over to another nitrogen.
In structure A, we have

Similarly, in structure B we have
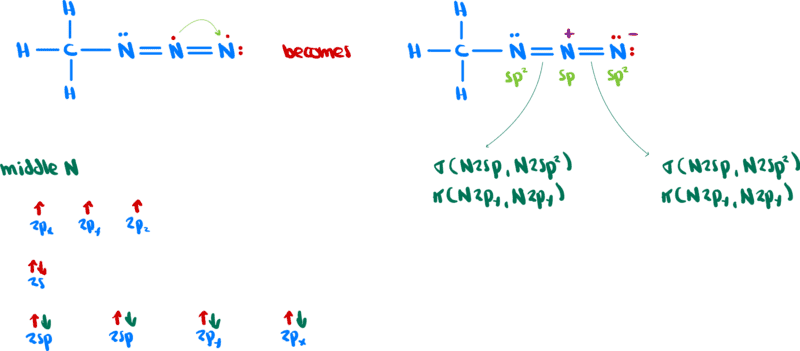
The middle nitrogen has the same hybridization in both structures. The difference is that in one case two ##\pi## bonds are formed with another nitrogen (for a triple bond) and in the other case there is one #\pi# bond with each nitrogen (for two double bonds).
My question, first of all, is if this analysis is correct.
Second of all, how do we know when a nitrogen will give up an electron as is happening here?
Now, I have a rudimentary understanding that in some models electrons are delocalized, but I am not aware that that is the case in valence bond theory.
After looking on Wikipedia, it seems the structure is
I am not sure about the reasoning that leads one to the above (resonant?) structures. Let's call the leftmost structure A and the rightmost structure B.
My question is what is the hybridization of the middle nitrogen and how do we think about the distribution of electrons among the molecular orbitals.
A nitrogen alone has the valence electron configuration
Now, in both the structures, there is are no unpaired electrons or any lone pairs on the middle nitrogen because, for some reason that I am unaware of, one of the electrons on the middle nitrogen goes over to another nitrogen.
In structure A, we have
Similarly, in structure B we have
The middle nitrogen has the same hybridization in both structures. The difference is that in one case two ##\pi## bonds are formed with another nitrogen (for a triple bond) and in the other case there is one #\pi# bond with each nitrogen (for two double bonds).
My question, first of all, is if this analysis is correct.
Second of all, how do we know when a nitrogen will give up an electron as is happening here?
Now, I have a rudimentary understanding that in some models electrons are delocalized, but I am not aware that that is the case in valence bond theory.
